C.W. Hand, Karen E. Ryan, Sujata K. Dutt, and R.A. Moore*
DPC European Research Institute, Station Lane, Witney, Oxfordshire OX8 6AN, United
Kingdom
J.O'Connor and Dymphna Talbot
Drug Centre and Toxicology Department, Jervis Street Hospital, Dublin 1, Ireland
H.J. McQuay
Oxford Regional Pain Relief Unit, Abingdon Hospital, Abingdon, Oxford, United
Kingdom
A radioimmunoassay kit (DPC buprenorphine double antibody)
was evaluated with clinical samples and samples from a drug
clinic. Urine samples were collected over a 2-day period
from 5 hospital in-patients receiving sublingual buprenorphine,
400 to 2000 µg/day, for the relief of chronic pain. Samples were measured
before and after enzymatic hydrolysis. Urine buprenorphine concentrations were
measurable at all doses studied (minimum value 5.6 ng/mL) and were greater with
larger doses. The increase in concentration after hydrolysis averaged 49% and
was similar for all doses studied. The authors conclude that the method has extensive
cross-reactivity with glucuronides of buprenorphine and its metabolites and that
samples may be analysed without prior hydrolysis. The prevalence of buprenorphine
use in 97 patients attending a drug clinic was also studied. Sixty (62%) had
measurable urinary buprenorphine concentrations of 1 ng/mL or more by direct
assay. The buprenorphine users were significantly younger and reported significantly
greater use of opiates than nonusers.
Buprenorphine is a powerful agonist analgesic
and is effective in treating many forms of pain (1). Its kinetics are
well understood (2). Despite influential findings
of low addiction potential (3) and the suggestion
that it may be useful in the management of opiate addicts (4),
there have been reports of misuse (5-7). Indeed, it
has been estimated that 50% of hardcore drug users in New Zealand
use buprenorphine (6), although this may reflect the
fact that buprenorphine is more easily obtained than are more
strictly controlled drugs. Impressions of the prevalence of buprenorphine
use, however, have not been validated by screening.A joint study
by the Medico-Social Research Board and the National Drug Advisory
and Treatment Centre5 or as it is commonly referred
to, the Jervis Street Drug Centre, of patients attending the
Drug Centre from 1979-1983 showed that during this five year
period there had been a six fold increase in the number of persons
making their first contact with the Centre and a four-fold increase
in re-contacts. Males exceeded females by a ratio of three to
one and the majority of attenders, approximately 60%, were aged
under 25 years. Opiates were the principal drugs of misuse and
in particular, heroin. Between 1979 and 1983 the numbers contacting
Jervis Street Drug Centre for the first time for treatment of
opiate misuse rose eight-fold from 56 to 451. This increase,
together with evidence from the Garda Drug Squad of a dramatic
increase in the number of persons charged for heroin-related
offences and of seizures of the drug, confirmed that there had
been an "opiate epidemic" in Dublin between 1979 and 1983.6 When
the attendance at the Jervis Street Drug Centre was analysed
for area of residence the attendance rates were highest from
north and south central Dublin. The Medico-Social Research Board
and the Jervis Street Drug Centre continued their collaboration
in a study of patients who had received treatment at the Centre
in 1984. This paper reports on the characteristics of the 1984
attenders making some comparisons with those of previous years
and also comments on information collected for the first time
in 1984. More recent information, as it is available, for 1985
and 1986 is also presented.
The plasma
kinetics of buprenorphine after intravenous, intramuscular,
and sublingual administration have been well studied over
a number of years (1,2,8). Excretion of buprenorphine and
its metabolites in urine have been less well studied. Using
GC/MS, Conet et al. (9) reported urinary excretion
of about 2, 13, and 12% of subcutaneous, sublingual and oral
doses respectively. No unconjugated buprenorphine was found,
and the urine excretion was as glucuronide conjugates of
buprenorphine and N-dealkylbuprenorphine with small amounts
of unconjugated N-dealkylbuprenorphine. In a single subject,
Blom et al. (10) found small amounts of buprenorphine
in the unconjugated form.
Immunoassay
is the preferred method for screening urine for buprenorphine
because chromatographic methods generally lack sensitivity (11).
This study was designed to examine a new urinary buprenorphine
radioimmunoassay kit, to determin urinary buprenorphine levels
in patients taking the drug for relief of chronic pain, and
to investigate the effects of hydrolysis on observed levels.
A small
pilot study employing the assay among patients in the Dublin
Drug Centre in early 1987 appeared to indicate a high incidence
of buprenorphine use (unpublished data). The study was therefore
extended to survey the drug centre population for the prevalence
of buprenorphine use.
Assay validation: The DPC buprenorphine
in urine double antibody kit was used as supplied
by the manufacturer (EURO/DPC Ltd.). N-Dealkylbuprenorphine
and etorphine were gifts from Reckitt & Colman
Pharmaceuticals Ltd. Controls containing 0,
5, and 15 ng/mL of buprenorphine (CONDOA-T, EURO/DPC) were
included in all assays. For testing cross-reactions in the
assay, drugs were made up in concentrations of 100,000 ng/mL
in drug-free human urine. N-Dealkylbuprenorphine and etorphine
(and other drugs that gave cross-reaction at 100,000 ng/mL)
were diluted until values on the standard curve were obtained;
buprenorphine-3-glucuronide and N-dealkylbuprenorphine glucuronide
were not available for testing.
Sample
hydrolysis: In order to determine whether buprenorphine-3-glucuronide,
N-dealkylbuprenorphine glucuronide (and possibly buprenorphine-3-sulfate)
are detected by the assay, samples from chronic pain patients
were subjected to enzymatic hydrolysis. For hydrolysis, patient
samples (100 uL) were diluted 1:1 with 0.01 mol/L citrate/phosphate
buffer pH 5.0 containing 200 units/mL and 80 units/mL respectively
of β-glucuronidase (EC 3.2.1.31, type H1 from Helix
pomatia, Sigma Chemical Co.) and Aryl sulfatase (EC 3.1.6.1,
type V, from Patella vulgata, Sigma).
Drug-free
human urine: Urine samples were collected from 50 volunteers
known not to be taking any drugs or medications.
Chronic
pain patients: Five patients (3 female) attending the pain
relief clinic were studied. All were taking buprenorphine
sublingually for relief of chronic pain; none had any clinically
apparent renal or hepatic disorder. The buprenorphine dose
was 400 to 2000 µg in divided sublingual doses, and
the dose of buprenorphine had not changed for at least 1
week before sample collection. Each patient provided urine
samples over a 2-day period while in hospital; a total of
25 samples were collected. Urine samples were frozen without
preservative until analysed.
Drug clinic
patients: In August 1987, patients attending the Jervis Street
Drug Centre gave the usual urine sample, and a form was completed
by the physician noting the age and sex of the patient, what
drugs they were accustomed to using, and whether they were
on methadone maintenance. Urine samples were screened for
the presence of drugs by thin-layer chromatography (TLC).
A buprenorphine test was taken as positive if the measured
concentration (on duplicate samples) was equal to or greater
than 1.0 ng/mL. Actual concentrations were recorded.
Statistical
differences between the ages of patients and the pattern
of drug use for those who were positive or negative for buprenorphine
in the urine were tested with the Mann Whitney U test or
the chi-square test (with Yates correction). In all cases
two-tailed tests of statistical significance were used. Mean
values and standard error of the mean are reported.
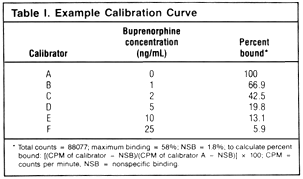
The buprenorphine assay gave a standard curve
between 1 and 25 ng/mL (an example assay is
shown in Table I). Interassay precision was
calculated from 20 individual assays; the zero
control always read below 0.1 ng/mL (by extrapolation). The
mean value obtained for the 5-ng/mL control was 4.8 ng/mL (coefficient
of variation (CV), 6.3%) and that for the 15-ng/mL control
was 15.2 ng/mL (CV 6.9%).
A
wide variety of drugs (Appendix A) was assayed at a concentration
of 100,000 ng/mL and found not to cross-react. Eight drugs
gave detectable readings in the assay. N-Dealkylbuprenorphine
gave a cross-reaction of 98% when tested at 10 ng/mL, and
etorphine gave a cross-reaction of 1.8% when tested at 100
ng/mL. Diprenorphine showed a maximum cross-reaction of less
than 0.1%; prednisolone; hydromorphone, dextromethorphan,
N-dealkyletorphine, and nalbuphine displayed less than 0.05%
cross-reaction.
Positive
urine samples diluted with zero calibrator gave results that
were parallel with the standard curve.
Drug-free
human urine: results for 50 drug-free human volunteers were
all below 1.0 ng/mL; the maximum observed concentration by
linear extrapolation of the standard curve was below 0.1
ng/mL.
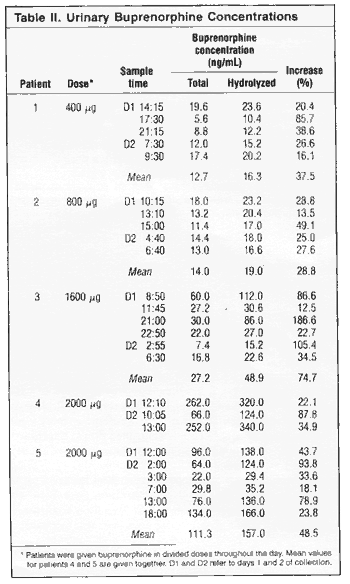
Chronic
pain patients. Results for individual patients and samples
are given in Table II. The lowest value found in a single
urine by direct assay was 5.6ng/mL. There were marked variations
in urine concentrations and in concentration increases after
hydrolysis (no dilution effect was apparent when diluting
samples in hydrolysis buffer without enzymes). Despite this,
the urinary concentration of buprenorphine was higher with
a higher daily dose of the drug, both for the direct assay
and after hydrolysis. The mean increase after hydrolysis
was 49% for all 25 samples, but the range was great - between
about 13 and 190% (median about 30%).
Drug clinic
patients: Complete records for 94 of the 97 patients studied
were obtained; all 97 results were included in the buprenorphine
analysis, and 94 were used for demographic analysis.
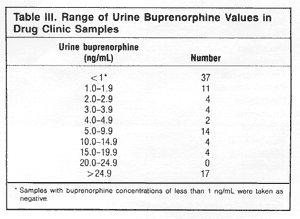
Measurable
urinary buprenorphine concentrations (1.0 ng/mL or greater)
were found in 60 (62%) of the 97 patients, and the number
of samples in different concentration ranges are shown in
Table III. Eleven patients had results between 1.0 and 1.9
ng/mL, and of these, only two had urine drug test positive
for other drugs (one each for opiates and benzodiazepines).
The demographic
data on the patients with buprenorphine-positive or negative
urine samples is given in Table IV. Those in the buprenorphine-positive
group were significantly younger (p<0.025, Mann Whitney U
test), but there was no difference in sex ration. Buprenorphine
users reported the use of nearly twice as many drugs as those
with negative urine test (p<0.001, Mann Whitney U test),
and this extra use was limited to opiate agonists (p>0.005)
and buprenorphine (P<0.025, chi-square test). The proportion
of patients on methadone maintenance or who had positive
urine tests for drugs other than buprenorphine or methadone
(predominantly benzodiazepines and opiates) were the same
in both groups.
Buprenorphine is a safe and effective analgesic,
and its abuse potential is usually considered
lower than that of other opiate agonists (3) because of its ability as an
antagonist to block the subjective effects of morphine and
because it does not produce physical dependence. The agonist
properties of buprenorphine, on the other hand, have suppressed
heroin self-administration in addicts (4) and have
induced buprenorphine self-administration in monkeys (12).
Lethal overdose appears to be impossible, however (14).
After sublingual
administration, buprenorphine plasma concentration rise slowly
and are maintained at a low concentration (1-3 ng/mL) for
some hours (14). Buprenorphine has a long half-life
of about 8 h (1,2), and the N-dealkyl metabolite appears
to have an even slower elimination (8). This accords
with the appearance of buprenorphine and its glucuronide
in urine in 1-2 days, and N-dealkylbuprenorphine and its
glucuronide of 1-4 days (9,10).
For detection
of buprenorphine in urine a test must be sensitive and must
measure not only buprenorphine but also its major metabolites.
The DPC double antibody buprenorphine assay has a calibration
range of 1 to 25 ng/mL and gives low coefficients of variation
at 5 and 15 ng/mL; apparent buprenorphine concentrations
in the urine of drug-free individuals were below 0.1 ng/mL.
The assay does not cross-react with a wide variety of drugs,
but does cross-react significantly with a one major metabolite,
N-dealkylbuprenorphine. The assay is considered to be sufficiently
specific for use as a urine screening test for buprenorphine.
The other
major urinary excretion products of buprenorphine, buprenorphine-3-glucuronide
and N-dealkylbuprenorphine glucuronide, were not available
for cross-reaction testing. Buprenorphine is excreted by
humans almost exclusively in conjugated form (9, 10).
If the assay detects glucuronides of buprenorphine and N-dealkylbuprenorphine,
enzymatic hydrolysis would be expected to make only a small
difference to apparent concentrations; if these metabolites
are not detected, then hydrolysis would be expected to increase
considerably the apparent urine concentration (by some 4
to 9 times). In the 5 patients studied here over a daily
dosage of 400 to 2000 µg buprenorphine, the mean increase
in observed concentration after hydrolysis was between 29
and 75%. The implication is that cross-reaction to glucuronides
was extensive.
Urine
buprenorphine values in patients administered sublingual
buprenorphine were all above the lowest calibrator in the assay.
Urine
buprenorphine concentrations were variable, but generally
increased with dosage. The minimum urine concentration
found was about 5 ng/mL by direct assay in a patient receiving
only 400 µg/day. Mean systemic availability of sublingual
buprenorphine is only about 65% (14), and so
400 µg sublingually is equivalent to 250 µg
given parenterally. This is less than a standard injected
analgesic dose of buprenorphine (and equivalent to about
7mg intravenous morphine). Measurement of urine buprenorphine
concentrations by radioimmunoassay should therefore detect
any illicit use of buprenorphine, although the data presented
here do not indicate how long after a dose buprenorphine
may be detected in urine.
In screening
97 patients from a drug clinic during a 1-month period,
the buprenorphine test indicated that 62% of the samples
had values greater than 1 ng/mL. This raises the question
of the appropriate cut-off value for the presence of buprenorphine
in urine. The extreme position of using 1 ng/mL, the concentration
of the lowest calibrator, was taken here. This is supported
by the specificity of the method, by the values of less
than 1 ng/mL obtained in drug-free individuals, and by
the similar frequency of methadone use and urine tests
positive for other drugs in the two patient groups (Table
IV). As drug testing guidelines evolve, different considerations
may determine other values. The proportion of positive
test at a cut-off of 1 ng/mL was 62%, and was 51%, 46%,
42% and 39% at 2, 3, 4 and 5 ng/mL respectively. A reliable
confirmation method for buprenorphine in urine is required.
In this
test, buprenorphine users were significantly different
from the group testing negative. They were younger, they
had experimented with more drugs (specifically opiates),
and 56% admitted using buprenorphine (compared with only
17% in the buprenorphine-negative group). This pattern
of buprenorphine use by hard-core drug users is similar
to that reported anecdotally in New Zealand and Edinburgh (6,7).
A major
reason for use of buprenorphine by experienced opiate addicts
attending drug clinics, apart form its opiate agonist properties
and availability, has been the virtual impossibility of
its detection in urine samples. The extremely low urine
concentration of buprenorphine and its metabolites make
detection by TLC highly unlikely, while HPLC also lacks
sensitivity (11) and GC/MS is time-consuming
and expensive. Implementation of drug clinic screening
for buprenorphine and its metabolites by immunological
methods should be an effective means of reducing the attractiveness
of buprenorphine use among opiate addicts.
We wish to tank Sister Dawn Carroll for
organising the collection of samples and
the ward staff of the Oxford Regional Pain
Unit of their assistance.
Compounds
that do not cross-react with the buprenorphine assay*
Acetaminophen
(Paracetamol)
Acetylsalicylic acid
Allylcyclopentyl barbituric acid
Alprazolam
Amitriptyline
Amobarbital
Amphetamine
Apomorphine
Aprobarbital
Barbituric acid
Bromazepam
Butabarbital
Butalbital
Butethal
Caffeine
Clorazepate
Chlorpromazine
Clonzaepam
Chlordiazepoxide
Cocaine
Codeine
Cotinine (nicotine metabolite)
Demozepam
Desmethyldiazepam
Dextroproproxyphene
5,5-Diallyl barbituric acid
Diazepam
Dihydrocodeine
Dipyrone
Dopamine
Ethyl morphine
Fenfluramine
Fentanyl
Flunitrazepam
Furosemide
Hexobarbital
Hydroxyamphetamine
Imipramine
Levamisole
Lidocaine
Lorazepam
Lysergic acid
Medazepam
Methlamphetamine
Methadone
Methaqualone
Morphine
Morphine-3-glucuronide
Morphine-6-glucuronide
Nalorphine
Nitrazepam
Normorphine
Nortirptyline
Oxazepam
Phencyclidine
Phenorbarbital
Phenylbutazone
Prednisone
Promazine
Reserpine
Secorbarbital
Temazepam
Triazolam
* These
compounds were undetectable in the buprenorphine assay
at a concentration of 100,000 ng/mL. Eight other compounds
were detectable by the buprenorphine RIA. Of these 6 had
cross-reaction of less than 0.1% (see text).
R.E.S.
Bullingham, J.J. McQuay, and R.A. Moore. Clinical pharmacokinetics
of narcotic agonist-antagonist drugs. Clin. Pharmacokinet.
8: 332-43 (1893)
H.J.
McQuay, R.A. Moore, and R.E.S. Bullingham. Buprenorphine
kinetics. In Advances in Pain Research and Therapy, K.N.
Foley and C.E. Inturrisi, Eds. Raven Press, New York,
1986, pp. 271-78.D.R.
Jasinksi, J.S. Pevnick, and J.D. Griffith. Human pharmacology
and abuse potential of the analgesic buprenorphine. Arch.
Gen. Psychiatry 35: 501-16 (1978).
M.K.
Mello and J.H. Mendelson. Buprenorphine supresses heroin
use by heroin addicts. Science 207: 657-59 (1980).
J.
Strang. Abuse of buprenorphine. Lancet ii: 725 (1985)
H.B.
Rainey. Abuse of buprenorphine. N.Z. Med. J. 134: 72
(1986)
J.R.
Robertson and A.B.V. Bucknall. BuprenorphineL Dangerous
drug or overlooked therapy. Br. Med. J. 292: 1465 (1986).
C.W.
Hand, D. Baldwin, R.A. Moore, M.C. Allen, and H.J. McQuay.
Radioimmunoassay of buprenorphine with iodine label:
Analysis of buprenorphine and metabolites in human plasma.
Ann. Clin. Biochem. 23: 47-53 (1986).
E.J.
Cone, C.W. Gorodetzky, D. Yousefnejad, W.F. Buchwald,
and R.E. Johnson. The metabolism and excretion of buprenorphine
in humans. Drug Metab. Dispos. 12: 577-81 (1984)
Y.
Blom, U. Bondesson, and E. Anggard. Analysis of buprenorphine
and its N-dealkylated metabolite in plasma and urine
by selected-ion monitoring. J. Chromatogr. 338: 89-98
(1985)
L.P.
Hackett, L.J. Dusci, K.F Iiet, S.S.W. Seow, and A.J.
Quigley. Sensitive screening method for bruprenorphine
in urine. J. Chromatogr 374: 400-404 (1986)
M.K.
Mello, M.P. Bree, and J.H Mendelson. Buprenorphine self-administration
by rhesus monkey. Pharmacol., Biochem. Behav. 15:215-25
(1981)
C.D.
Banks. Overdosage of buprenorphine: Case report. N.Z.
Med. J. 89: 255-56 (1979)
R.E.S.
Bullingham, H.J. McQuay, E.J.B. Porter, M.C. Allen, and
R.A. Moore. Sublingual buprenorphine used postoperatively:
Ten hour plasma drug concentration. Br. J. Clin. Pharmacol.
13: 665-73 (1982)

